|
Sol-Gel Materials and Nanotechnology Center
of Excellence
 
Address: ul. Smoluchowskiego 25
50-370 Wrocław, Poland
phone: (+48 71) 320 21 18
fax: (+48 71) 321 12 35
Email: sgmn@immt.pwr.wroc.pl
Introduction
Poland and other countries aspiring to full membership in the
European Community have to bridge many gaps in a very short time.
The discrepancies between them and the members of the Community
span almost every aspect of life - from the law to pollution of
water, soil and atmosphere. Yet, there is an enormous potential
in these future members of the EU - given a chance their young
generations can join the greater family of the European nations
bringing new ideas, skills and enthusiasm.
Wrocław - the city in which the Center
is localized - is uniquely positioned where Poland, Germany, the
Czech and Slovak Republics meet. Furthermore, it is a great
academic center - 11 higher-education establishments with total
enrolment of 111,000 students (17% of its population). As such
Wrocław is a perfect candidate for a hub of scientific and
technological exchange between the East and the West of Europe.
Establishing of the Center of
Excellence "Sol-Gel Materials and Nanotechnology" in
Wrocław was aimed primarily at creating an international network
of research groups (originating from universities, national labs
and other research-oriented establishments), companies interested
in new technologies and materials as well as local-government
institutions and integrating this network into the greater
European Research Area. That networking would occur in several
intersecting areas by building contacts: between scientists from
different fields (interdisciplinary research), between
scientists and manufacturers (technology transfer) and
between scientists/manufacturers and local communities (response
to local economic and social challenges and needs). The above
mentioned networking between scientific, industrial and
governmental institutions would cover as broad area of Europe as
possible, helping in this way to incorporate the participant
from the NAS into the European Community.
Objectives
The particular objectives envisaged for the "Sol-Gel
Materials and Nanotechnology" Center of Excellence cover:
networking - organizing and coordination of the network
of scientific, industrial and governmental institutions aimed at
enhancing of cooperation between them and yielding joint projects
leading to: obtaining grants, publishing scientific papers,
formulating patents and organizing spin-offs; contacts with other
Centers specializing in related topics will be established and
twinning proposals will be formulated
organization of international and local conferences and
workshops - stimulating exchange of scientific and
technological ideas, bringing together people from different
areas and countries
organizing short-term visits for senior and junior
scientists - helping in establishing contacts aimed at
formulating new research projects capable of obtaining funds from
local sources and the European Commission (e.g. the framework
programs)
publishing a scientific journal - the Center is already
involved in preparing, editing, printing and providing via the
internet of "Materials Science". The journal - which
covers various aspects of new, advanced materials - is a perfect
medium for achieving increase in the scope and impact of the
envisaged network.
Innovative character
New, smart materials and nanotechnology belong to the topics
showing the fastest rate of growth and immense impact on
contemporary science and technology. Due to the advances of
experimental and computational techniques in physics and
chemistry it is possible now to obtain direct information about
molecular and atomic structure of matter (nanometric scale)
and dynamics of physicochemical processes governing its behavior
observed in real-time (femto- and nanosecond scale).
These methods include:
supramolecular chemistry - enabling the
researches to build desired macroscopic structures by controlling
the formation process (chemical recontent/sgmn/actions) on the
nanoscale (e.g. carbon nanotubes (fullerenes),
electroluminescent organic displays etc.)
fast lasers - pulsed lasers operating in nano-
and femtosecond regimes are used to investigate and modify
elementary physicochemical processes enabling researchers to
obtain materials with completely new properties (e.g. quantum
dots, nonlinear optics etc.)
optoelectronics - optical fibers, optical
amplifiers, new generations of detectors are used to integrate,
miniaturize and enhance systems of information collection,
transmission and evaluation yielding more powerful tools for
science and technology
scanning tunneling microscopes (STM) and atomic force
microscopes (AFM) - research tools enabling
visualization of structures of virtually any objects on nanoscale
spectroscopic techniques - such methods as NMR,
UV-VIS-IR absorption techniques, Raman scattering,
luminescence measurements, ESCA etc. yield fast and reliable
information on composition and properties of materials under
investigation.
The above mentioned tools and techniques are just a few of a
fast growing family of techniques enabling researches to
conceive, build, test and apply new advanced materials which
structure and properties are controlled on the molecular and
atomic levels.
The sol-gel technique is one of the fastest growing fields of
contemporary chemistry. The main advantage of this process stems
from the fact that it offers an alternative approach to
conventional production of glasses, glass-like materials and
ceramics of various properties and applications. Conventional
glass preparation requires melting of the precursors at high
temperatures, rapid cooling and subsequent vitrification of the
glassy material. This procedure highly restricts choice of
substances which can be entrapped in the glass products.
Furthermore, the way in which conventional glass is produced
makes thin films preparation extremely cumbersome. Production of
porous glassy materials via the conventional melting is similarly
inconvenient.
The sol-gel technology enables
production of doped glassy materials either as porous dry gels ("xerogels")
or densified materials. Since the process starts from aqueous
solutions of precursors it is possible to immobilize in
glass-like materials various substances even as fragile as
proteins (yielding bio-content/sgmn/active glasses).
Furthermore, contrary to the conventional methods of glass
production it is possible to obtain doped materials with
extremely large dopants concentrations - a crucial feature in
production of, for example, nonlinear optics based on rare-earth
doped glasses and ceramics (e.g. erbium fiber-optical
amplifiers for telecommunication industry). Another
attrcontent/sgmn/active feature of this technology is the fact
that sol-gel materials can be obtained as bulks, thin films (on
various supports) and powders.
Various materials obtainable by the sol-gel technique and the
processes leading to their production are shown in below:
Bulks 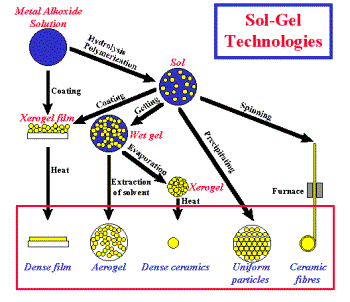
Sol-gel technologies
The SGM&N Center of Excellence is
content/sgmn/actively involved in designing, production,
investigations and applications of various materials of the
sol-gel origin. Examples of the main thematic areas:
optical sensors (optodes) - the idea is based on
changes of optical parameters of content/sgmn/active (sensing)
molecules physically entrapped in (or, in some cases, covalently
bound to) porous sol-gel thin films (on planar or fiber-optical
supports). Those changes are induced by changing external
physico-chemical parameters such as temperature, hydrostatic
pressure or presence of analyte molecules or, for example,
bacteria [e.g. a Gold Medal with Distinction during
the 48th World Exhibition
of Innovation, Research and New Technologies Eureka'99 for
"sol-gel optode of gaseous ammonia"]
non-linear optoelectronics - the sol-gel
technique is used to obtain densified glasses and ceramics doped
with high concentrations of, for example, rare earth
content/sgmn/active ions [e.g. a Gold Medal with
Distinction during the 49th
World Exhibition of Innovation, Research and New Technologies
Eureka'00 for erbium doped sol-gel preforms for fuber-optical
amplifiers]
nanomaterials - e.g. nanopowders consisting of
uniform spheres of submicron sizes; such spheres can further
modified with various content/sgmn/active substances yielding
materials exhibiting, for example, the photonic effect (e.g.
for optical integrated circuits)or several orders of
magnitude increase in intensity of Raman signal (e.g. for
medical biosensors based on the SERS efect).
The Center of Excellence is coordinated through the Management
Board. The role of the Management Board is to:
· develop long-term plans concerning the Center
strategic research directions,
· obtaining financial support and expand the
Center capabilities
· control the Center financial status
· coordinate contacts with industry and local
governments
· coordinate the staff policy and stimulate the
staff development.
The Board consists of:
- the Scientific Coordinator
- the task leaders
- editor-in-chief of "Materials Science"
The members of the Board have proven scientific
qualifications. Each of them possesses also leadership qualities.
The Center of Excellence Scientific Coordinator is Prof.
Krzysztof Maruszewski.
The working groups with their leaders are dedicated to
carrying specific tasks:
- Scientific projects - organization of work,
coordination and content/sgmn/active participation in
preparation of grant proposals, spin-offs and other
research-oriented content/sgmn/activities. The working
group will be also responsible for obtaining scientific
results, preparation of papers, reports, patents etc. - Leader
1 - Prof. Wiesław Stręk
- Events - work concerned with all aspects of
organization of conferences, workshops and related events
- Leader 2 - Prof. Marek Rybaczuk
- Information - preparation, actualization and
providing of databases and the Center official web page.
Also, preparation of the Center booklets and maintaining
contacts with the Center partners - Leader 3
- Prof. Jerzy Kaleta
- "Materials Science" journal - editing
and publishing of "Materials Science" - person
responsible - Prof. Juliusz Sworakowski Editor-in-Chief
(Institute of Physical and Theoretical Chemistry,
Technical University of Wrocław)
- The Center office - secretarial support of the
Center of Excellence - person responsible – Dr.
Katarzyna Kozłowska, the Center secretary.
The information flow within the Center core is coordinated by the
Center office. The Scientific Coordinator is
responsible for introducing measures decided upon the Management
Board. The Management Board decisions are subject to
the Supervisory Board advises and revisions. The
Management Board meets every month (or more frequently, if
need arises). The Management Board measures and plans are
consulted with the Supervisory Board every three month.
The Center of Excellence is a structural part of the Technical
University of Wrocław. As such, its infrastructure and working
environment is a part of the University macrostructure. Safety
concerns as well as equal opportunity regulations are thus
warranted by the standing rules of the University. As a public
higher-education facility the Technical University of Wrocław
conforms to the Polish Law and international academic
regulations.
Supervisory Board
The supervisory Board guides and advices the Management Board
in its efforts in leading, coordination and expanding of the
SGM&N Center of Excellence.
The Supervisory Board consists of:
1. Prof. M.A. Aegerter, Institut für Neue Materialien - INM,
Germany
2. dr. J. Czyżewski,ABB Corporate Research, Poland
3. Prof. J. Holsa, University of Turku,Finland
4. Prof. A. Kłonkowski, University of Gdańsk, Poland
5. Prof. J. Legendziewicz,Wrocław University, Poland
6. eng. R. Okniński, KOLT S.A. Technical-Commerce Firm,
Poland
7. Prof. M. Opałło, Institute of Physical Chemistry of the
Polish Academy of Sciences, Poland
8. Prof. J. Phalippou, Laboratoire des Verres - UMR 5587,
Universite de Montplier 2, France
9. Prof. R. Reisfeld,The Hebrew University of Jerusalem,
Israel
10. Prof. M. Bettinelli, Universita degli Studi di Verona,
Italy.
Background information on SGM&N Coordinator

Krzysztof Maruszewski (Ph.D. D.Sc.)
He was born 08.08.1964 in Zielona Góra, Poland. He graduated
from the Faculty of Basic Problems of Technology of Wrocław
Technical University in 1988. His M.S. Thesis "Computer
Simulations of Side Directed Mutagenesis and Is Influence on
Proteins content/sgmn/activity and Stability" was
prepared in the Quantum Chemistry Group under a guidance of prof.
dr hab. inż. A. Sokalski and resulted in publication in Int.
J. Quant. Chem. As a student he was a chairmen of
theAssociation of Biotechnology Students (1987-1988) where he
organized the Ist and IInd National
Biotechnology Seminars. He received three Chancellor of Wrocław
Technical University Awards (1985, 1986, 1988) and Minister of
Higher Education Award ("Primus Inter Pares") in 1987.
He begun his doctoral study at Marquette University,
Milwaukee, WI, USA in the group lead by prof. J. Kincaid in 1989
. During his graduate study at Marquette University he completed
five proficiency exams in the first year of the program and
received: Dennis J. O'Brien Fellowship (1990), Rev. J.P. Raynor,
S.J. Fellowship (1991-1992) and "Certificate of
Accomplishment" in 1994. He obtained his Ph.D. degree
in 1992 for Thesis: "Spectroscopy and Physicochemical
Properties of Ruthenium(II) Polypyridine Complexes in Free
Solutions and Entrapped in Zeolites". He continued
research at Marquette University as an Associate Researcher
during his post-doctoral work (1992-1995).
After return to Poland in 1995 he begun to work in the
Institute of Low Temperature and Structure Research (Polish
Academy of Sciences) in Wrocław and in the Chemistry Department
of Opole University. The Scientific Council of the Institute
granted him a D.Sc. degree for "Physicochemical and
Spectroscopic properties of metal complexes and organic molecules
immobilized in zeolites and sol-gel matrices" in June of
1999. He was nominated for an Assistant Professor position
in the Institute of Low Temperature and Structure Research in
March of 2000. In the same year he was also offered a
professor position at the Wrocław University of Technology
which he accepted (ending my work at the Opole University).
In 1997 a Polish Scientific Research Committee (KBN)
grant "Technology and Physicochemical Properties of Optical
Chemi- and Biosensors (Optodes) Based on Sol-Gel Silica
Matrices" (of which he was a Principal Researcher) was
nominated to the "Polish Nobel" series. The
results of the grant were evaluated as "excellent"
and described in several newspapers and TV programs. At the
present moment he is a Principal Researcher of another three KBN
grants concerned with such devices. He also took part in five
other KBN grants. He was a member of a Coordinating Committee of
a SCI-TECH II grant (program Phare) related also to optical
sensors.
He was awarded:
- a Gold Medal with Distinction during the 48th
World Exhibition of Innovation, Research and New
Technologies Eureka'99,
- a Gold Medal with Distinction during the 49th
World Exhibition of Innovation, Research and New
Technologies Eureka'00,
- an Individual Prize of the Polish Minister of Education
in 2000.
He took part in 8 international conferences and 23 conferences
in Poland. He is an author of 44 published publications and two
books. He has six submitted patents. Number of citations: 178
(1989-2001). He is a supervisor of eight PhD students. He was
an organizer of ten conferences including chairmanship of the
"Sol-Gel Materials 2001" International Conference
(Rokosowo, Poland, 2001). He is a member of the Polish Chemical
Society.
International attrcontent/sgmn/action
The SGM&N Center of Excellence possesses significant, well
proven research capabilities in the field of theory,
manufacturing, investigations and applications of a variety of
sol-gel based materials, including nanomaterials.
The major experimental capabilities of
the Center are based, among others, on the following labs:
· several synthetic labs - full synthetic
capabilities (chemical benches, high-temperature furnaces,
dry-boxes etc.)
· chemical analytical labs - various methods
(electrochemical, chromatographic etc)
· microbiological and biochemical labs -
techniques for biological sol-gel sensoric system
· microscopy labs - Transmission Electron
Microscopes, Scanning Electron Microscopes, various optical
techniques
· lasers lab - complete coverage of optical
laser methods (e.g. argon lasers, krypton lasers, an excimer
laser, a dye laser, a YAG:Nd, a Ti:Sapphire laser etc.)
· spectroscopy lab - UV-VIS-IR absorption and
emission techniques (from liquid-helium to high-temperatures),
Raman scattering, excited states lifetime measurements etc.
· crystallography lab - single-crystal and
powder techniques
· material fatigue lab - various materials
fatigue tests
· computational facilities - the Technical
University Computational Center
The Center has established links with many scientists
representing various fields from Europe and other parts of the
world. Examples of the scientific institutions from which
researchers have visited the Center include:
· Prof. M.A. Aegerter, Institut für Neue
Materialien – INM, Germany
· Prof. V. Zolin, Institute of Radioeneering
and Electronics of the Russian Academy of Sciences, Russia
· Prof. J. Phalippou, Laboratoire des Verres -
UMR 5587, Universite de Montplier 2, France
· Prof. N.V. Gaponenko, Belarusian State
University of Informatics and Radioelectronics, Belarus
· Prof. J. Holsa, University of Turku, Finland
· Prof. R. Reisfeld, The Hebrew University of
Jerusalem, Israel
· Prof. R. Acevedo, Ciudad Universitaria,
Santiago, Chile
· Prof. G. De Sa, Cid. Universitaria, Recife,
Brazil
· Prof. T. K. Anh, Inst. Material Sciences,
Hanoi, Vietnam
· Prof. S. Bratccini, Univ. Modena & Reggio
Emilia, Modena, Italy
· Prof. L. C. Klein, Rutgers State University,
USA
· Prof. D. Millers University of Latvia, Riga,
Latvia
· Prof. H. C. Zeng,National University of
Singapore, Singapore
and many others.
The Center has been visited by many students and post-docs,
among others, from: Finland, France, Germany, China, Vietnam,
Belarus and Italy.
The visits, exchanges and other forms of international
cooperation are possible due to the fact that the scientific
staff of the Center of Excellence communicates (in speech and
writing) in English. Furthermore, French, German and Spanish
speakers are among the Center Polish participants. The senior
scientists of the Center of Excellence possess wide experience in
international contacts (degrees obtained at the foreign
universities, memberships of international societies,
participations in conferences in different parts of the World).
Currently, the SGM&N co-operates with the Network of
Centers of Excellence "Interfacial Effects, Novel Properties
and Technologies of Nanostructured Materials" (Acronym
NANOstructured materials) in developing of nanostructured
ceramics for optoelectronics applications.The Center was
represented during the II Workshop of the Network of Centers of
Excellence (5-6 October 2001, Ulm Germany).
Sol-gel technology - Introduction
The sol-gel technology emerged within the last two decades and
quickly became one of the most important and promising new
material fabrication methods. It enables researches to design and
fabricate a wide variety of different materials with unique
chemical and physical properties. The sol-gel materials are based
on silica, alumina, titania and other compuonds. The sol-gel
technology allows to fabricate: monolithic and porous glasses,
fibres, powders, thin films, nanocrystalllites, photonic
crystals. A broad spectrum of different sol-gel derived materials
opens a variety of new applications in a broad range of fields:
optoelectronics (optical sensors, lasers, filters, photonic
crystals), ferroelectronics, luminophores, scintillator ceramics,
cathodoluminescent screens, powder lasers, anti-reflection
coatings, superconductors, telecommunication (optical fibers and
wave guides), medicine (bio-glasses), catalysis, anticorrosion
coatings, new chemicals for agriculture, pharmacy (controlled
drug delivery systems).
The following technology themes are under current development
in the Center of Excellence: fabrication of porous monolithic and
thin film glasses, fabrication of glasses doped with transition
metal and rare eart ions, fabricontent/sgmn/action of optical
sensors (optodes) of various physical and chemical parametres,
fabrication of nanometric crystallites, fabrication of pure and
doped submicron spherical particles, fabrication of
inorganic-organic materials hybrid.
The sol-gel technique
Conventional glass preparation requires melting of the
precursors at high temperatures, rapid cooling and subsequent
vitrification of the glassy material. This procedure highly
restricts choice of substances, which can be entrapped in the
glass products. Basically only metal oxides and some inorganic
salts can survive such drastic conditions avoiding thermal
decomposition. Furthermore, the way in which conventional glass
is produced makes thin films preparation extremely cumbersome and
the only method of preparation of porous classical glasses
requires etching or partial dissolving (e.g. Vycorâ glass). On
the other hand glass and glassy materials possess several useful
features for optical applications such as transparency,
homogeneity, mechanical sturdiness, high refrcontent/sgmn/active
index etc.
An alternative approach to glass and glass-like materials is
offered by the, so called, sol-gel technology. The process itself
is known for more than a century, but it has gained a new
importance in the last two decades after pioneering results of
Dislich. He and other researchers improved the chemistry of the
process so much that it is now possible to obtain samples in days
(or even hours - in case of thin films) rather than months (or
years) like in the case of the early samples.
The sol-gel technique is based on hydrolysis of liquid
precursors and formation of colloidal sols. The precursors are
usually organosilicates (e.g. TEOS - tetraethoxysilane) yielding
silicate sol-gel materials. However, the method is not restricted
to the silicon compounds - for example compounds of zirconium,
vanadium etc. can be used as precursors leading to materials
possessing different physico-chemical properties. Furthermore, it
is possible to obtain modified organosilicate precursors with
direct Si-C bonds (which do not undergo hydrolysis) and
possessing terminal functional groups (e.g. -NH2, -SH2 etc.).
Such precursors, either pure or mixed with the conventional ones,
yield inorganic-organic materials with mechanical (e.g.
elasticity) and physico-chemical properties (e.g. wetability)
modified by the organic components of the inorganic polymer
network. The functional groups can be also used for covalent
binding of various chemicals (including biomolecules) giving
specifically modified glassy materials.
In the case of the most often employed silicate sol-gel
matrices, manufactured from hydrolizates of various
alkoksysilanes, the chemical recontent/sgmn/actions involved in
the gel formation involve the precursor hydrolysis and the
subsequent formation of the silicate network.
At this stage a wet gel is produced which, upon drying, yields
porous xerogels. The drying is accompanied by liquid expulsion
from the pores (syneresis) and substantial matrix shrinkage often
leading to cracks (mainly due to the capillary pressure).
The hydrolysis process is significantly improved (accelerated)
if conducted at pH 7. Thus, addition of acid (typically HCl(aq))
or base (typically NH4OH) speeds up this process.
After the hydrolysis the acidity of the sol is neutralized slowly
to approx. 7 pH, what stimulates the gelation process. At this
stage a mechanically unstable "wet" gel is formed.
Drying of wet gels (even at ambient temperatures) leads to
xerogels ("dry gels"). Xerogels are stable, transparent
and insoluble in water and most of organic solvents and porous
solid materials.
In cases when fully-densified sol-gel glasses are sought,
extensive drying at temperatures close to the vitrification
temperatures will yield such materials (e.g. for silicate glasses
it is necessary to heat at quartz vitrification temperature
Vg&asymp1300°C). This enables obtaining glasses in
situations when it is not feasible via the conventional melting
techniques (e.g. heavily-doped with certain temperature-resistant
materials).
Since the early steps of the sol-gel process occur in liquid
phase, it is possible to add basically any substance (as
solutions or suspensions) at this stage. Simple mixing provides
uniform distribution of the dopant within the liquid host phase.
After the gelation the guest molecules become physically
entrapped within the now solid host matrix. Furthermore, the
hydrolysis, doping and gelation occur usually at ambient
temperatures - allowing entrapment of even such delicate
molecules as proteins without their decomposition. Sol-gel doped
matrices, obtained in the above described manner, are of the form
of xerogels and possess a network of internal pores and cavities
enabling the entrapped molecules to interact with the surrounding
medium. Furthermore, the doped matrices usually possess good
optical characteristics (transparency and high
refrcontent/sgmn/active indexes). Those features are of key
importance for production of optical sensors (optodes).
Submicron silica spheres prepared by the
sol-gel method
Another convenient feature of this technology is the fact that
the sol-gel samples can be obtained as bulks, thin films and
powders. It has to be noted that bulk sol-gel samples suffer very
often from internal cracks, leading to their destruction. This
effect is caused by evaporation of solvent molecules from the
network of pores of the drying gels. The ensuing capillary
pressure is high enough to cause the material collapse. However,
for reasons only partially understood, sol-gel thin films are
virtually immune from this destructive effect. Thus, for example
sol-gel optodes based on such thin films possess all the
attrcontent/sgmn/active features of the sol-gel materials being,
at the same time, virtually free from the most troublesome
drawback of the method i.e. samples cracking.
The sol-gel technique is one of the fastest growing fields of
contemporary chemistry. The main advantage of this process stems
from the fact that it offers an alternative approach to
conventional production of glasses, glass-like materials and
ceramics of various properties and applications. The sol-gel
technology enables production of doped glassy materials either as
porous dry gels ("xerogels") or densified materials.
Since the process starts from aqueous solutions of precursors it
is possible to immobilize in glass-like materials various
substances even as fragile as proteins (yielding
bio-content/sgmn/active glasses). Another attrcontent/sgmn/active
feature of this technology is the fact that sol-gel materials can
be obtained as bulks, thin films (on various supports) and
(nano)powders. Such matrices, content/sgmn/activated by doping,
impregnation or covalent bonding, yield materials which can be
used as, among other possibilities, optical sensors, catalysts,
medical materials (e.g. bone implants), content/sgmn/active (e.g.
"smart windows") and passive coatings (e.g.
scratch-resistant or antireflective) or various optical materials
(e.g. scintillators, powder lasers, amplifiers etc.).
References:
[1] Ebelman, H. (1847). C. R. Acad. Sci., 25, 854.
[2] Dislich, H. (1971). Glastechn. Ber., 44, 1.
[3 Wolfbeis, O.S. (Ed.) (1991). Fiber Optical Sensors and
Biosensors, CRC Press, Boca Raton, Florida.
[4] Maruszewski, K., Strommen, D.P. and Kincaid, J.R. (1993). J.
Am. Chem. Soc , 115, 8345.>br/> [5] Maruszewski K. and
Kincaid J.R. (1995). Inorg.Chem., 34, 2002.
[6] Maruszewski, K., Andrzejewski, D. and Stręk, W. (1997). J.
Luminesc., 72-74, 226.
[7] Maruszewski, K., Jasiorski, M., Salamon, M. and Stręk, W.
(1999). Chem. Phys. Lett., 314, 83.
[8] Lakowicz, J.R. (Ed.) (1994). Topics in Fluorescence
Spectroscopy Vol. 4: Probe Design and Chemical Sensing,Plenum
Press, New York.
[9] Marazuela, M.D., Moreno-Bondi, M.C. and Orellana, G. (1998).
Appl. Spectrosc., 52, 1314.
[10] Campbell, M., Yang, Y., Wallace, P.A. and Holmes-Smith, A.S.
(1997). Opt. Rev., 4, 111.
[11] Nakamoto, K. (1978). Infrared and Raman Spectra of Inorganic
and Coordination Compounds, John Wiley, New York.
[12] Clark, R. J. H. and Hester, R. E. (1978 up to now). Advances
in Infrared and Raman Spectroscopy.
[13] Long, D. A. (1977). Raman Spectroscopy McGraw-Hill, New
York.
[14] Strommen, D. P., (1984). Laboratory Raman Spectroscopy, John
Wiley, New York.
[15] unpublished results.
[16] Stöber, W., Fink, A. and Bohn, E. (1968). J. Colloid
Interface Sci., 26, 62.
[17] Jasiorski, M., Maruszewski, K. and Stręk, W. (2002).
Mat.Sci., 20, 51.
[18] Maruszewski, K., Jasiorski, M., Hreniak, D., Stręk, W.,
Hermanowicz, K. and Heiman, K. J. Sol-Gel Sci.Techn., in press.
[19] Campion, A. and Kambhampati, P. (1998). Chem. Soc. Rev., 27,
241.
[20] Li, X.Y., Petrov, V.I., Chen D. and Yu, N.T. (2001). J.
Raman Spectr., 32, 503.
[21] Kurokava, Y., Imai, Y. and Tamai, Y. (1997). Analyst, 122,
941.
[22] Litorja, M., Haynes, C.L., Haes, A.J., Jensen T.R. and Van
Duyne, R.P. (2001). J. Phys. Chem. B, 105, 6907
Optical fiber sensors and luminescence
biosensors
prepared by the sol-gel method
The idea behind the sol-gel optical sensors (optodes) is based
on changes of optical parameters of content/sgmn/active (sensing)
molecules physically entrapped in (or, in some cases, covalently
bound to) porous sol-gel thin films. Those changes are induced by
changing external physico-chemical parameters such as
temperature, hydrostatic pressure or presence of analyte
molecules or, for example, bacteria. There are several kinds of
optical signals which could be used as analytical response of
such optodes. In general, one can observe changes of:
- time of decay of the sensing molecules luminescence,
- intensity of light absorbed or emitted by the sensing
molecules
- changes in vibrational spectra (bands intensity and
frequency) of the sensing molecules,
- polarization properties of the sensing molecules.
Electronic spectroscopy in the visible region offers several
advantages stemming from the fact that relatively small number of
inorganic, organic or metalloorganic molecules possesses
electronic transitions in the VIS range (color). This fact lowers
the possibility of optical interference with the sensing
molecules coming from unwanted molecules (in most cases organic),
which might diffuse into the matrix pores or adhere to the optode
surface. Furthermore, spectral bands corresponding to such
transitions are usually broad in condensed phases (liquids and
solids) and, often, even in gases. This property makes them
easier to detect. Simple colorimetric measurements of acidity
supply an example of this analytical method. In this case changes
in pH result in changes of absorption patterns (color) of organic
indicator dyes such as, for example, phenolophtaleine,
bromocresol, rosolic acid etc.
As it has been mentioned, optodes can be based on detection of
changes of polarization properties of the sensing molecules bound
to sol-gel matrices. For example, it is possible to covalently
bind large, biological molecules labeled with luminophors to the
surface of a sol-gel thin film in such a way that the
macromolecule retains partial rotational freedom. If some other
molecule recognizes and binds to the labeled biomolecule its
rotation can be hampered what leads to changes of the luminophore
emission polarization anisotropy. The system based on the this
effect can be employed as a luminescent sensor for detection of
certain bacteria.
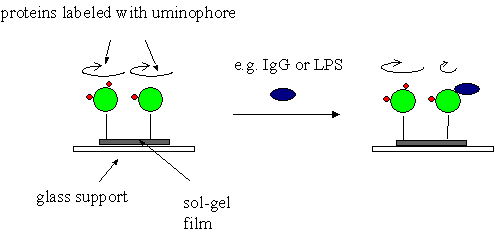
The luminescence biosensor based on
detection of changes of depolarization of luminescence
Thin films of titania and silica-titania
composites
New methods of fabrication of the thin films obtained by the
sol-gel method for bacteriostatic and optical applications have
been developed. Both types of materials are prepared from
hydrolyzed alkoxides of silicon and titanium (or their mixtures).
The well-known antiseptic properties of silver are due to
influence of silver ions on various microorganisms. Silver ions
are produced by oxidation of metallic Ag in the air. It is
related to the fact that this process yields a thin layer of AgO
on the metallic core of particle. Thus, it is important to
prepare materials with well dispersed silver particles,
possessing high specific surface and nanometric metal
precipitates. These requirements are met by the sol-gel derived
materials. Easy preparation of thin films and the simple process
of silver doping allows to prepare antiseptic and non-toxic for
humans materials for medical applications and the food industry.
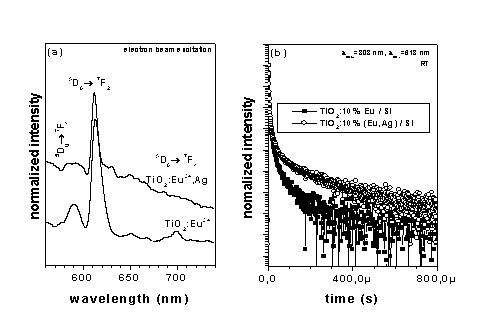
Emission spectra (a) and luminescence
decays (b) recorded for TiO2 doped with
Eu3+ and co-doped with Eu3+
and silver particles
Besides antiseptic properties of silver doped materials, a
strong influence of metallic, nanostructured Ag on the
luminescence efficiency of light emitting lanthanides ions, which
are generally used as luminophores, has been found recently. This
effect is due to phonon-plasmon intercontent/sgmn/action which is
present in dielectric materials doped with nanosized metallic
particles under optical excitation. The luminescent thin films of
nanocrystalline TiO2 content/sgmn/activated with
Eu-ions (red luminophore) and co-doped with silver particles have
been successfully obtained by the sol-gel method and their
emission properties have been investigated (Fig.1). The effect of
silver doping on the luminescence lifetimes increasing, which
indicates on the increase of emission efficiency from Eu3+-ions,
has been found. The fabricated material can be used as a new,
planar luminophores for optoelectronic applications (for instance
in Field Emission Displays (FEDs)).
Magnetic Powders
Understanding the correlation between magnetic properties and
(nano)structure involves collaborative efforts of chemists,
physicists and materials scientists to study both fundamental
properties and potential applications. Magnetic nanoparticles of
a single magnetic domain size possess very interesting properties
and the potential to replace many of the conventional magnetic
materials.

Ferromagnetic materials, magnetic powders, have been widely
used as magnets, cores and wave absorbers.
Nanoparticles of magnetic materials are used in magnetic
disks, recording tapes, etc.
Superparamagnetic nanoparticles also find important medical
applications e.g. magnetic cell sorting, magnetocytolysis,
drug-targeting experiments etc.
The correlation between nanostructure and magnetic
properties suggests the classification of nanostructures
morphologies:

Type A
Materials include the ideal ultrafine particle system, with
interparticle spacing large enough to approximate the particles
as nonintercontent/sgmn/acting. The isolated particles with
nanoscale diameters.

Subgroup of Type A
Materials in which magnetic particles are
surrounded by a surfactant preventing intercontent/sgmn/actions.
Ferrofluids, in which long surfactant molecules provide
separation of particles.
Type B
Materials include ultrafine particles with a core-shell
morphology. In type-B particles, the presence of the shell can
help to prevent the particle-particle intercontent/sgmn/actions.

Type C
Nanocomposites are composed of small magnetic particles
embedded in chemically dissimilar matrix. The matrix may or may
not be magnetocontent/sgmn/active.
 
Type D
Materials consists of small crystallites dispersed in
noncrystalline matrix. The nanostructure may be two-phase, in
which nanocrystalites are a distinct phase form the matrix, or
the ideal case in which both the crystallites and matrix are made
of the same material.
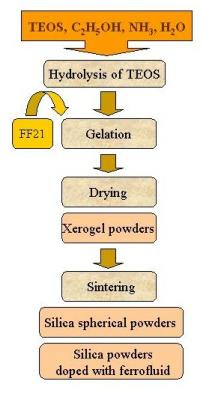
The spherical powders doped with ferrofluid and other magnetic
compounds are prepared by sol-gel process, modified in the part
of TEOS hydrolysis technique. Spherical hydrous silica particles
with narrow size distribution were previously produced by
Stöber, et al. via the hydrolysis/condensation method. Under
conditions of constant stirring at room temperature, TEOS
(tetraethylorthosilicate) was added to a solution of
reagent-grade ethanol and concentrated ammonium hydroxide
solution. The TEOS underwent hydrolysis/condensation
recontent/sgmn/action, forming (poly)silicic acid. Within
minutes, precipitation of uniform spherical particles occurred.
Figure 1. Diagram of the sol-gel preparation procedure for SiO2
powders doped with the magnetic fluid (FF21)
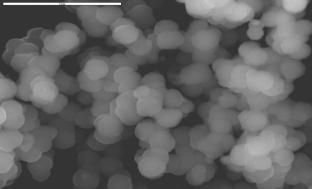
Figure 2. SEM photographs of spherical magnetic powders.
Organic - inorganic polymer hybrid

 An organic-inorganic hybrid
polymer hybrid materials has been obtained by combining organic
termo- and photopolymerization and the sol-gel process. The
organic and inorganic phases are formed from two interpenetrating
polymeric networks. The family of organic–inorganic hybrid
materials have attracted considerable attention due to their
interesting properties such as: molecular homogeneity,
transparency, flexibility and durability. Such hybrids are
promising materials for various applications, e.g.: solid state
lasers (optical components), replacements for silicon dioxide as
insulating materials in the microelectronic industry,
anti-corrosion and scratch resistant coatings, contact lenses or
host materials for chemical sensors, membrane materials and many
medical and dental applications, such as: dental filling
materials. Other areas of applications are catalysis,
chromatography. The potential applications and technologies are
limited due to their unique properties and characteristics. An organic-inorganic hybrid
polymer hybrid materials has been obtained by combining organic
termo- and photopolymerization and the sol-gel process. The
organic and inorganic phases are formed from two interpenetrating
polymeric networks. The family of organic–inorganic hybrid
materials have attracted considerable attention due to their
interesting properties such as: molecular homogeneity,
transparency, flexibility and durability. Such hybrids are
promising materials for various applications, e.g.: solid state
lasers (optical components), replacements for silicon dioxide as
insulating materials in the microelectronic industry,
anti-corrosion and scratch resistant coatings, contact lenses or
host materials for chemical sensors, membrane materials and many
medical and dental applications, such as: dental filling
materials. Other areas of applications are catalysis,
chromatography. The potential applications and technologies are
limited due to their unique properties and characteristics.
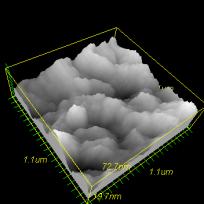
Figure 2. AFM micrograph of SiO2/acrylamide-HEMA copolymer
hybrid.
Organic-inorganic hybrid materials are synthesized by the
sol-gel process. The sol–gel process is an interesting approach
to preparation of the inorganic phases in the organic–inorganic
hybrid materials due to the fact that it can occur in liquid
solutions at room temperature. The sol–gel technology provides
an excellent way of obtaining transparent and mechanically stable
films, bulk glasses and powders. Furthermore, it provides a
convenient way of producing porous materials. The general sol-gel
recontent/sgmn/action is based on hydrolysis of various alkoxides
to form respective silanols. This is followed by a condensation
recontent/sgmn/action occurring between silanols or silanols and
alkoxides. The sol-gel process involves evolution of inorganic
networks through the formation of a colloidal suspension (sol)
and gelation of the sol to form a network in a continuous liquid
phase (gel). The precursors for syntheses are used alkoxysilanes
such as tetraethoxysilane (TEOS) or alkoxytitanium such as
titanium tetrabutoxide (TBT). However, alkoxide such as silica
and titanate are also commonly used in the sol-gel process to
form mixed-presursor inorganic matrices. We are functionalized
surface inorganic materials by vinyl, phenyl, methyl, ethyl and
epoxy group.
The organic precursor such as
acrylamide or 2-hydroxyethylmethacrylate (HEMA) or mixture of
these monomers were introduced to the liquid hydrolyzed silicate
solutions. The compositions silica/organic monomers were
irradiated by UV light at room temperature. The organic polymer
and copolymer phase have been prepared by radical polymerization
with benzil used as a photo-radical initiator. The organic
polymer also are prepared via thermopolymerization. The obtained
materials have been investigated by spectroscopic (IR, Raman),
microscopic (SEM, AFM) and thermal analysis (TGA, DTA)
techniques. The N2-adsorption were used to
characterize the textural properties such as: specific surface
areas (SBET), pore volumes (Vp), average
pore sizes (Rp) and micropore volumes (VDR).
The complete adsorption-desorption isotherms and pore size
distributions were analyzed following the Dollimore-Heal method.
The textural parameters was measured for organic-silica hybrid
materials and for materials after pyrolysis. The pyrolysis of the
inorganic-organic polymer hybrid samples was performed at 600°C
under oxygen flow. The textural parameters was measured for
organic-silica hybrid materials and for materials after
pyrolysis. The pyrolysis of the inorganic-organic polymer hybrid
samples was performed at 600°C under oxygen flow. All
organic-inorganic polymer hybrid samples are non-porous
materials. However after complete pyrolysis at 600oC
all samples show high microporosity .The result of the
pyrolysis silica show that pyrolysis of the sample leads to
mezoporous material. Contact angle vs. water (Langmuir balance),
hardness (Vicer's method), roughness (AFM) were also measured.
Organic phase is composed of organic hydrophobic and amphyphilic
acrylate homo- and copolymers. 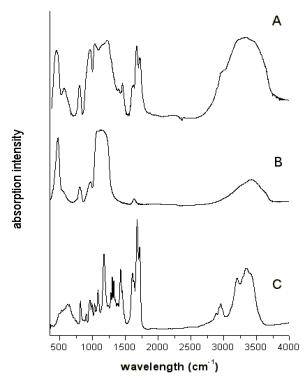 However, we have obtained
hydrophilic organic-inorganic interpenetrating However, we have obtained
hydrophilic organic-inorganic interpenetrating
polymer network. Figure 3 presents FT-IR spectrum of the SiO2/acrylamide-HEMA
copolymer hybrid, sol-gel SiO2 bulk and free organic
copolymer. The spectra show that is possibility to obtain
organic-inorganic polymer hybrid by the sol-gel method and the
organic photo-polymerization method. The picture of the hybrid
bulk sample (Figure 1) shows that the samples are transparent and
do not exhibit irregularities which might indicate such phase
separation.


At present, we have investigated structure and properties of
hybrid SiO2-TiO2-organic polymer xerogels
obtained by the sol-gel method. We have the intention to describe
the preparation, the chemical structure and the mechanical
properties of the organic-inorganic silica-titanium polymer
hybrid. Further our research area will be synthesis and
properties of titanium-based organic-inorganic polymer hybrid
xerogels.
We have developed organic–inorganic polymer hybrid for
photo- and electroluminescence, which we can used as organic
light emitting diode (OLED). These materials we have obtained by
the sol-gel method. The basic of classic organic light-emitting
diode is a Metal (e.g.: indium tin oxide) – Electroconductive
polymer (e.g.: polypyrole) – Electroluminescent material
(e.g.: organic rare earth compounds) - Metal (e.g.: aluminium
or calcium). In our work electroconductive polymer and
electroluminescent material have been interpenetrated in
inorganic sol-gel materials. Porous inorganic sol-gel film have
been good hole layer – injecting and transporting layer. OLED
have attrcontent/sgmn/active properties such as high
luminescence, low driving voltage, easy fabrication of large
areas and a wide range of emission colors.


Sol-gel materials under gamma rays
Gamma rays which are generated by radiocontent/sgmn/active
materials, have the smallest wavelengths of any other wave in the
electromagnetic spectrum. They are very penetrating and can
kill living cells. Therefore, they are used in medicine to kill
cancerous cells or in food industry to sterilization. Gray is the
SI unit of absorbed dose of radiation. The gray is the absorbed
dose when the energy per unit mass imparted to matter by ionizing
radiation is one joule per kilogram (1 Gy = 1 J/kg).
In our research, influence of gamma rays on organic molecules
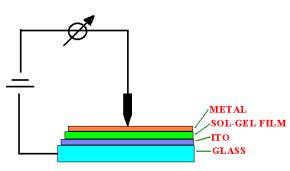
entrapped in sol-gel matrices is investigated. Tetraethoxy- and
tetramethoxysilan (TEOS and TMOS) are used to obtain the
matrices. The compounds enclosed in porous silicate bulks are
dyes with strong absorption band in the visible region (metal
phthalocyanines, complexes of ruthenium(II) or safranine). Dyes
are dissolved in water or organic solvent and added to the sol.
The obtained matrix shows very good transmission properties in
the investigated region even after irradiation. The translucent
bulks are exposed to 60Co radiation source at room
temperature, at a dose rate of 15,8 Gy/min. For comparison, the
solutions of the dyes are also irradiated.
When the dye dissolved in solvent is exposed to gamma
radiation, the decay of absorbance is observed. The rate of the
decay depends among others on a kind of solvent, exposure time,
concentration of the dye and the level of oxygen. When, for
example, magnesium(II) phthalocyanine (MgPc) dissolved in
dimethyl formamide (DMF) is exposed to irradiation, gamma rays
cause the complex decomposition. This effect is registered as the
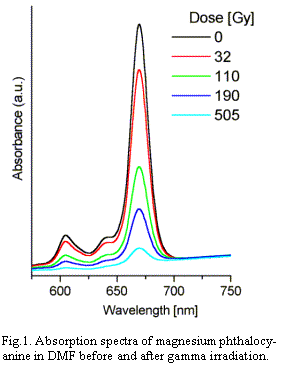
discoloration of the solution and the decay of the absorption.
The complex has three absorption bands in the visible region
(~ 604, 642 and 670 nm). The fast decrease in the absorbance is
observed for all of them (Fig.1). The initial blue color almost
disappears at doses of about 1 kGy (for the initial
concentration of the dye 0,62 mM). The decay of absorbance at
670 nm versus dose or gamma irradiation is exponential.
Neodymium bisphthalocyanine (NdPc2) in DMF has two
absorption bands in the visible region (~ 630 and 670 nm) which
can be assigned to the monomeric and dimeric form of the
phthalocyanine. The fall in absorption depends on the solvent.
For the complex in DMF, at the beginning of irradiation, the
longer-wavelength band increases and the other decreases its
intensity. The monomer : dimer ratio changes during
irradiation. Probably the dimer form degradation is faster. In
DMF : H2O solution the ratio is constant at the
beginning. The faster decrease in the dimer form seems to appear
in the later stage of radiation. In one case the decay of
absorbance versus dose or gamma irradiation is exponential, in
the other case, it is linear.
Gamma rays also changes the properties of silicate matrix. It
becomes nontransparent in the blue region of electromagnetic
spectrum at big doses. The absorption shifts from 270 to
400&minus450 nm for 1000 kGy. The bulks, from transparent
and colorless, becomes yellowish. The changes are quite similar
for bulks from TEOS and TMOS.
 The absorption
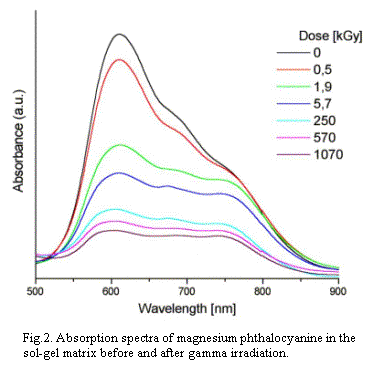
spectra of phthalocyanines in silicate matrix are different as
compared to the solutions. For MgPc, there is a broad band with
maxima at 610, 680 and 750 nm. The decay of absorption intensity
under gamma rays is much slower in the xerogel (Fig.2). It slows
a lot especially in the later stage of irradiation. Even up to
radiation dose of 1100 kGy, the bulk samples are still color and
the sol-gel matrix has very good transmission properties in
the investigated region. Further research
were difficult because the bulk started to disintegrate. The absorption
spectra of phthalocyanines in silicate matrix are different as
compared to the solutions. For MgPc, there is a broad band with
maxima at 610, 680 and 750 nm. The decay of absorption intensity
under gamma rays is much slower in the xerogel (Fig.2). It slows
a lot especially in the later stage of irradiation. Even up to
radiation dose of 1100 kGy, the bulk samples are still color and
the sol-gel matrix has very good transmission properties in
the investigated region. Further research
were difficult because the bulk started to disintegrate.
For NdPc2 in the sol-gel matrix the broad
absorption band consists of at least three overlapping bands.
They also can be assigned to the monomeric and aggregated forms
of the Pc. First doses of radiation causes fast degradation.
After abut 40 kGy the degradation is much slower. The blue
shift, broaden and increase of the band intensity is observed.
The longer-wavelength band is assigned to monomer. It is possible
that faster degradation of this form causes apparent shift and
the absorption increase. The change in the monomer&harrdimer
equilibrium is also possible. The variation in the absorbance
depends on the concentration of the dopant. For different
concentrations, the shift and absorbance growth appear at the
beginning or in the later stage of irradiation.
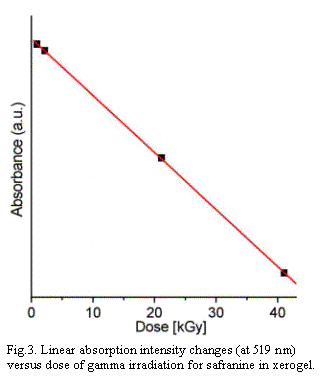
To apply the sol-gel matrix with organic compounds as a
gamma radiation sensor, it should show proper dependence of
absorbance on gamma dose. Only in some cases the dependence is
linear or, more often, exponential. Such relationship can be
found only for some range of doses. Usually, the straight line
can be drawn for small doses and the curve line for larger doses.
Perhaps some molecules could be applicable to the dosimeters
purpose. For instance, safranine which shows linear decay in a
large range from 0 to 40 kGy (Fig.3) or
tris(1,10-phenantroline)ruthenium(II) with exponential
decay from 0 to 150 kGy.
As a result of intercontent/sgmn/action between matter and
ionising radiation, radicals
and solvated electrons are formed. They can later react
with molecules in the environment. The radiation induced
transformation of complex compounds are:
– redox processes leading to the change of
oxidizing degree of central ion which changes the coordination
number and causes the molecule decomposition;
– radicals effect (recontent/sgmn/actions with
radicals);
– recontent/sgmn/actions with excited molecules of
solvents.
But the final products of the decomposition of many molecules
are unknown. The radicals formed under high-energy radiation can
be responsible for the degradation. The macrocyclic rings are
especially sensitive
to radicals attack. The cleavage of carbon&minuscarbon,
carbon&minushydrogen and carbon&minusnitrogen bonds is
possible in the dyes molecules.
The decrease in the dye decomposition
rate in the sol-gel matrix might be related to lower
concentration of free radicals formed in it upon gamma radiation.
Another possibility is that the matrixinduced change of the
macrocycle complex surroundings occurs which might increase the
dye stability.
The main conclusions of the research are:
– gamma rays cause decomposition of organic
molecules entrapped in sol-gel matrix.
–
– the dye decomposition rate is lower in the
sol-gel matrix in comparison with dyes in solutions.
– the linear or exponential decay in absorbance
occurs only in a particular range of doses.
– in the xerogel less radicals can be formed or
the matrix increases the dye stability.
Silica, titania and silica-titania powders
Silica (SiO2), titania (TiO2) and
silica-titania (SiO2-TiO2) powders have
been prepared by sol-gel method using silicate precursors:
tetraethoxysilane (TEOS), phenyltriethoxysilane (TPhOS),
vinyltriethoxysilane (TVOS), (3-Aminopropyl)-triethoxysilane
(APTES) and titanium precursors: titanium(IV) ethoxide (TEOT),
titanium(IV) buthoxide (TBOT) and titanium(IV) isopropoxide
(TIPO). Silicate and titanium precursors were dissolved in
ethanol, butanol and isopropanol. The recontent/sgmn/actions were
catalyzed by ammonia. Various SiO2, TiO2
and SiO2-TiO2 powders can be produced
depending on the solvent/precursor ratio and the solvent used
during the hydrolysis. Morphologies of the obtained powders were
characterized by the Scanning Electron Microscopy (SEM) and
Transmission Electron Microscopy (TEM). The sol-gel process has
been used as a synthetic route to obtain silica and titania
particles with spherical morphology. The results are presented
in Figures 1-4.
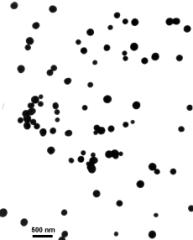 A A
 B B
 C C
 D D
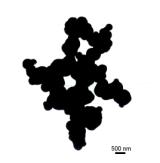 E E
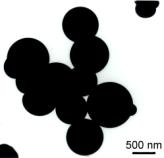 F F
Fig. 1. TEM micrograph of silica powders prepared from TEOS
(A-E) and APTES (F).
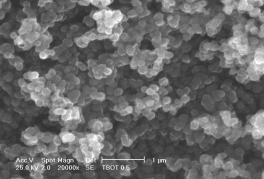 A A  B B
Fig. 2. SEM micrographs of titania powders
prepared from TBOT (A,B)
 A A 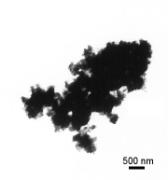 B B
Fig. 3. SEM and TEM micrographs of titania
powders prepared from TIPO (A,B).
 A A 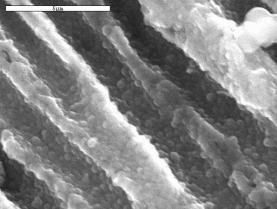 B B
Fig. 4. SEM micrographs of silica-titania
powders: TPhOS/TEOT (A) and TVOS/TEOT (B).
Some of the obtained titania powders possessed spherical
grains. The grains size and shape depends on the synthetic
conditions and the precursors used. The smallest grain sizes were
observed for the recontent/sgmn/actions with TIPO and the biggest
for the TBOT syntheses. The important parameter controlling the
particle formation and aggregation was the water/precursor molar
ratio.
TG-DTA and X-ray powder analysis
Titanium dioxide TiO2 has three naturally occuring
crystalline structures: anatase, brookite and rutile. The rutile
form is thermodynamically most stable at standard conditions. The
metastable anatase and brookite forms can be transformed to
rutile by heating. The sol-gel synthesized titanium powders are
amorphous in nature and the crystalline structure is achieved
only after heat treatment. The temperature needed for the
crystallization is dependent on the recontent/sgmn/action
conditions and the preparation route. The exothermic peak around
4000C (Fig. 5) is related to the phase-transformation
to rutile. The X-ray diffractogram of the titania powders shows
the diffrcontent/sgmn/action pattern characteristic to rutile
crystalline phase (Fig. 6). No peaks characteristic to anatase
and brookite appear in powders heated at 5000C.
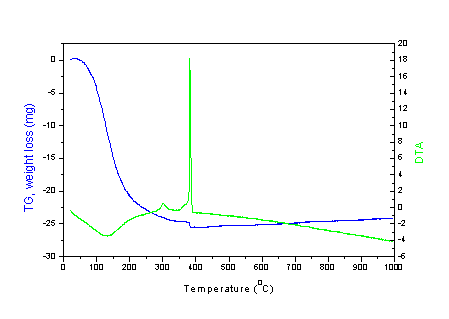
Fig. 5. TG-DTA curves of titania powdres
prepared by TEOT.
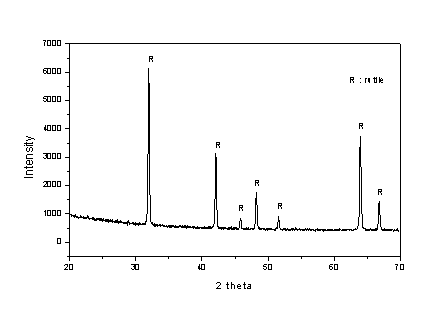
Fig.6. XRD patterns of titania powders prepared
from TEOT and calcinated at 5000C.
Molecular imprinting
Molecular imprints are obtained by introduction of inorganic,
organic or biological molecules during the polymerization step.
Subsequent removal of the molecules leaves stable vacant imprints
and designed sites for the recognition of the template molecules.
Materials obtained in this way are ideally suited for
applications such as separations, chemical sensing and catalysis.
Surfaces of silica particles were molecularly imprinted with
organic molecules: safranin, rodamine B, rosolic acid. Silica
particles were prepared in the presence of ethanol solution of
these molecules. Organic molecules were removed by calcination.
The resulting imprinted particles show adsorption of organic
molecules. The molecular adsorption of the surface-imprinted
particles was larger than in the case of particles without
imprints.
content/sgmn/activity progress report of the year 2003
The Center is effectively expanded and broaden its scope.
· First of all, the Center web page has been established
(http://sgmn.immt.pwr.wroc.pl/). It contains information about
the Center profile, its content/sgmn/activities, the coordinating
Institutions (the Wrocław Technical University and the Institute
of Low Temperature and Structure Research, Polish Academy of
Sciences), the management, link to "Materials Science"
etc.
· The Center has applied and been accepted to the International
Sol-Gel Society (www.isgs.org) and is listed at and linked to
the largest international portal dedicated to the sol-gel
science: The Sol-Gel Gateway (www.solgel.com/Research/resgr.htm).
· The Center has presented its content/sgmn/activities at the
largest Polish Fair – the International Poznań Fair, Poznań,
Poland (mtp.mtp.pl). Our presentation took form of a stand
(#66/Pavilion 14) dedicated to the Center and presented during
the forum: Science for the Economy (a part of the 75th
Fair of Industrial Technologies and Investment Goods) on
9-12.06.2003. There were, among other materials presented,
available for the Fair participants: posters, booklets and CD
discs pertaining to the Center content/sgmn/activities.
· The scientists participating in the Center have
content/sgmn/actively took part in basic and applied research in
the field of sol-gel materials and nanoscience. One of the major
achievements is the Gold Medal at the 52nd
World Exhibition of Innovation, Research and New Technologies
"Eureka" in Brussels (November 2003) received for
"Nanocomposite Materials for content/sgmn/active
Textiles". The submicron silica spheres doped with
nanoclusters of metallic silver has been used for
content/sgmn/activation of textile coatings giving them
antibacterial capability.
· The scientists affiliated with the Center has published a
total of 16 scientific papers in 2003.
The Center has expanded its contacts with other groups working
in the field. These contacts will lead to enhancement of the
Center experimental capabilities, create new opportunities for
fruitful personnel exchanges and lay ground for joint grant
proposals directed to the 6th Framework Program of the
EC. Already, three proposals were submitted to the 6th
Framework Program in 2003. During 2003 scientists affiliated with
the Center submitted several proposals to the Polish Ministry of
Science and Informatization. Two of them have been successful.
The financing of the grants for development of intelligent
textiles based on various doped nanopowders obtained by the
sol-gel method and for the sol-gel–based optical sensors
(optodes) has started with beginning of 2004.
A seminar entitled "The
"Sol-Gel Materials and Nanotechnology" Center of
Excellence - an Overview" was presented, among others:
during the 2nd International Conference "Sol-Gel
Materials", Rokosowo, Poland, June 2003, during the XII
Organosilica Compounds Seminar, Dymaczewo, Poland, October 2003,
at the Vilnius University, Vilnius, Lithuania, November 2003 and
the Institute of Radio Engineering and Electronics of the Czech
Academy of Sciences, Praque, the Czech Republic, December 2003.
The Center content/sgmn/activities were also presented in
several press articles and TV programs. Also, a series of
lectures on new developments in nanotechnology was organized for
high school teachers of physics.
The international journal "Materials
Science" after several-years long break restarted its
operation with the first issue of the completely changed volume
20 at the beginning of 2002. "Materials Science" is
published by the Wrocław University of Technology, in
collaboration with the Institute of Low Temperatures and
Structure Research of the Polish Academy of Sciences and the
Wrocław University of Economics. The journal is accessible in
two ways: the conventional printed form and in the electronic
form. Thus, full texts are available instantly after manuscripts
acceptance (and before the paper version printing) at
www.MaterialsScience.pwr.wroc.pl. This ensures short waiting
times and quick information delivery to scientists worldwide.
During 2003 the journal expanded its operation and scope and the
electronic database has been improved. The high quality of the
scientific papers published and the journal regularity resulted
in "Materials Science" being added to the ThompsonO
ISI Journal Data Base (the "Philadelphia list" at
www.isinet.com/journals) in the autumn of 2003.
SGM2004 is an interdisciplinary meeting
aimed as a platform for information exchange between
prcontent/sgmn/actically and theoretically oriented scientists
interested in materials obtained by the sol-gel technique. The
Second International Conference on Sol-Gel Materials
"Research, Technology, Applications" SGM'03 took place
on 15 - 20 June 2003 in Szklarska Poręba, Poland
(http://smartsite.immt.pwr.wroc.pl/index/SolGel). This second in
a row event gathered around 100 participants from various
countries (Belarus, the Czech Republic, England, Finland,
France, Germany, Israel, Italy, Japan, Lithuania, Poland and
Spain) - doubling its size since the First SGM Conference in
Rokosowo, Poland organized in 2001
(http://www.immt.pwr.wroc.pl/sgm2001). The Conference has covered
a vide field of topics related to various aspects of theory and
prcontent/sgmn/actice of the sol-gel technology.
Some of SGM&N Awards
   
The SGM&N Team:
Prof. Krzysztof Maruszewski
Prof. Wiesław Stręk
Dr. Marek Jasiorski
Dr. Katarzyna Kozłowska
mgr inż. Dariusz Hreniak
mgr inż. Agnieszka Hreniak
mgr Róża Kornak
mgr Iwona Zaręba-Grodz
mgr inż. Anna Łukowiak
mgr Beata Borak
|